Endovascular
Devices and Complications Learning Series
Stents and Scaffolds
Episodes
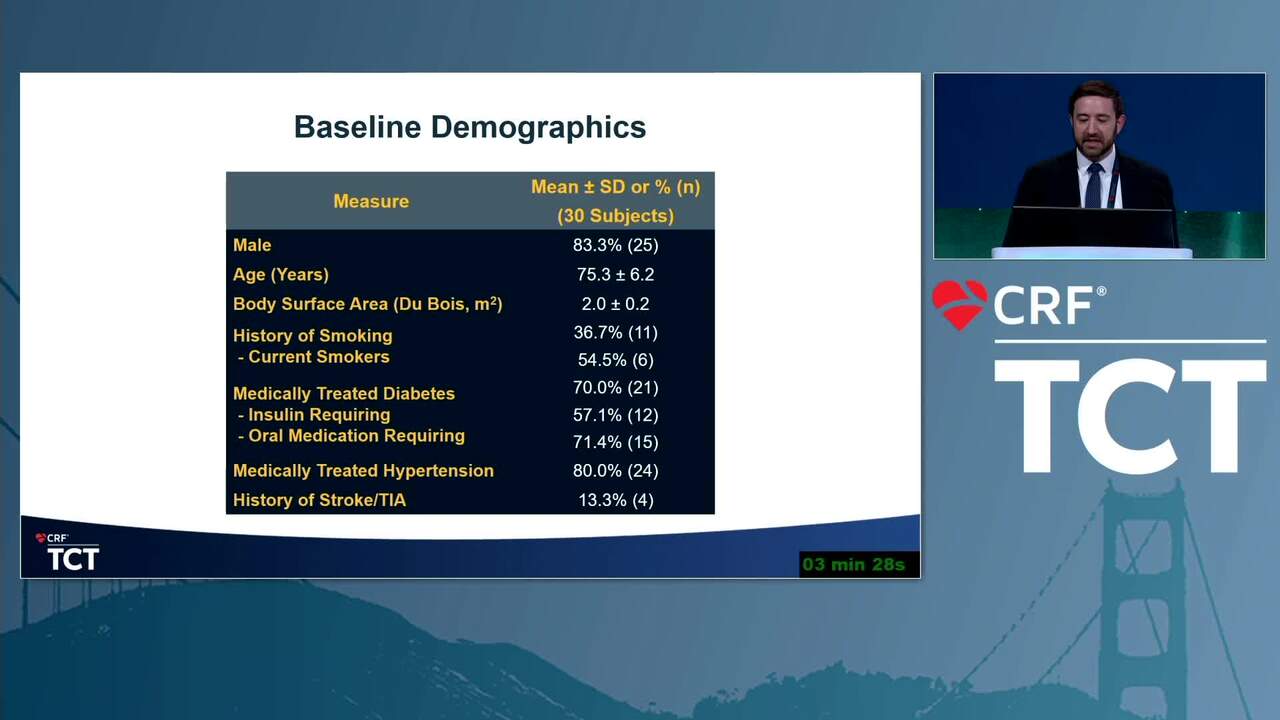
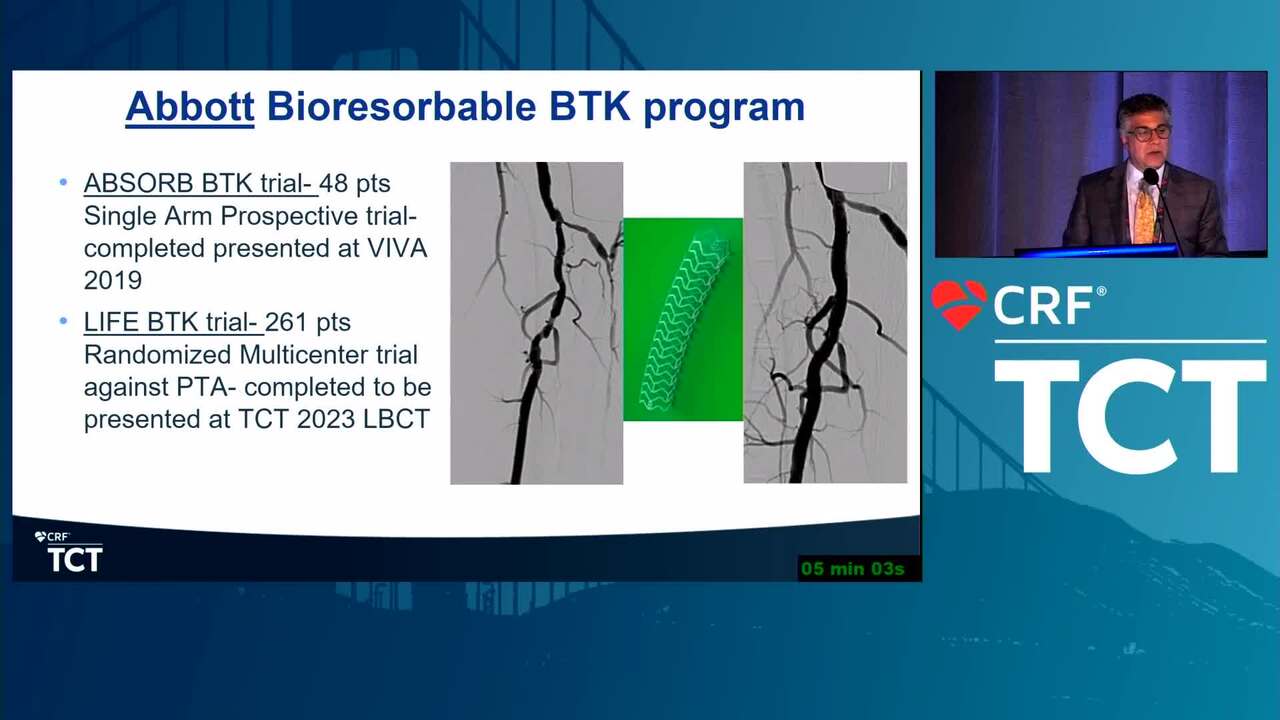
Primary Outcomes of the Esprit™ BTK Drug-Eluting Resorbable Scaffold for the Treatment of Infrapopliteal Lesions: The LIFE-BTK Randomized Controlled Trial
21 min.
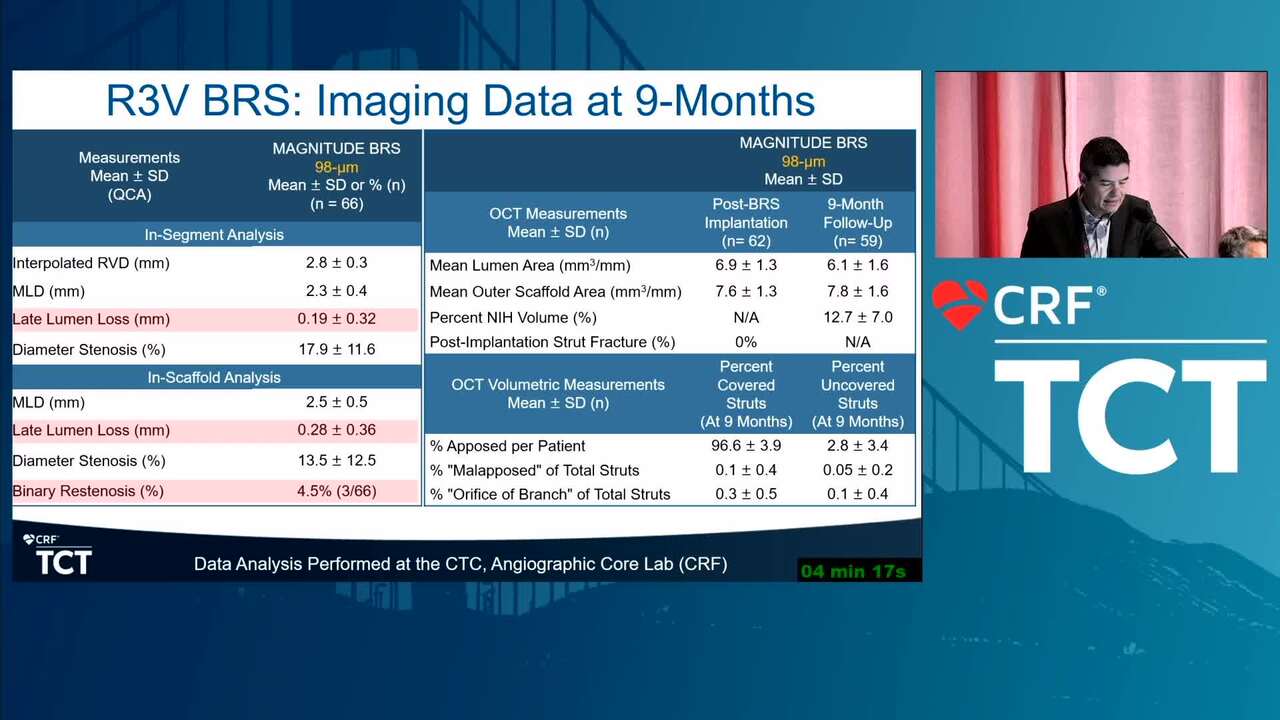
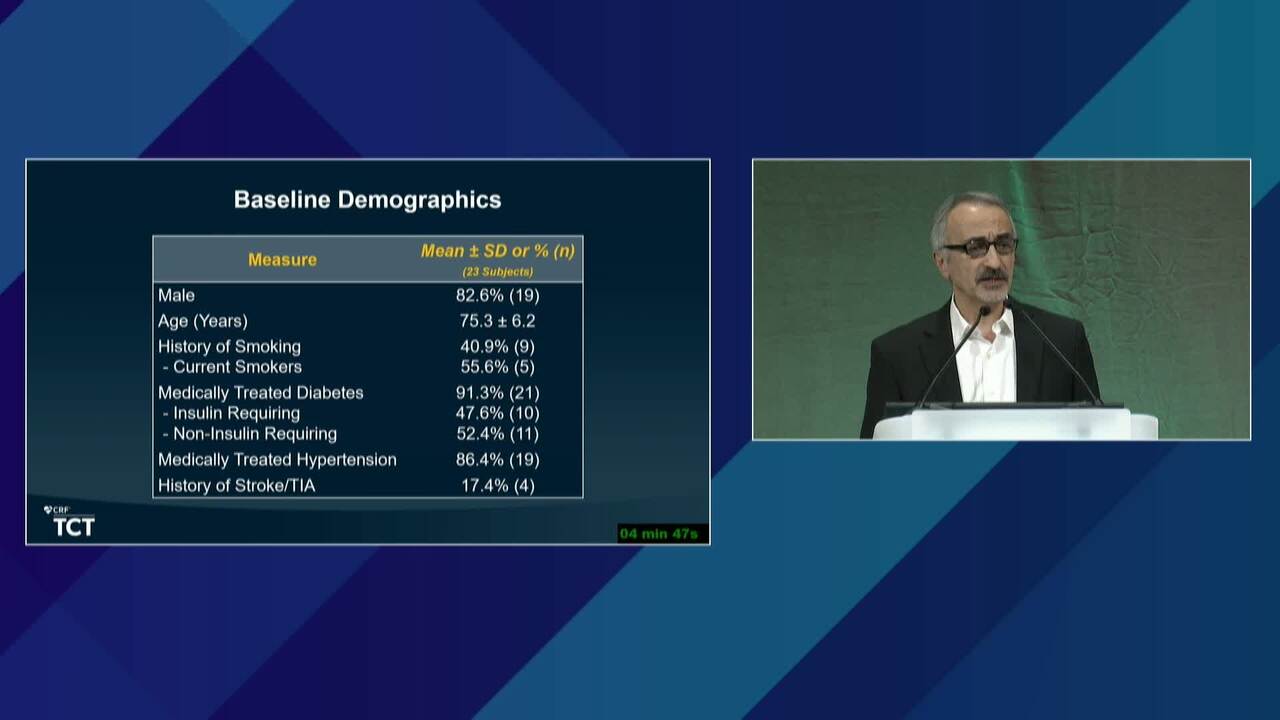
Angiographic and Clinical Outcomes of the Thin-Strut MAGNITUDE Bioresorbable Scaffold in Patients with Below-the-Knee Arterial Disease: Early Results from the RESOLV I Trial
9 min.
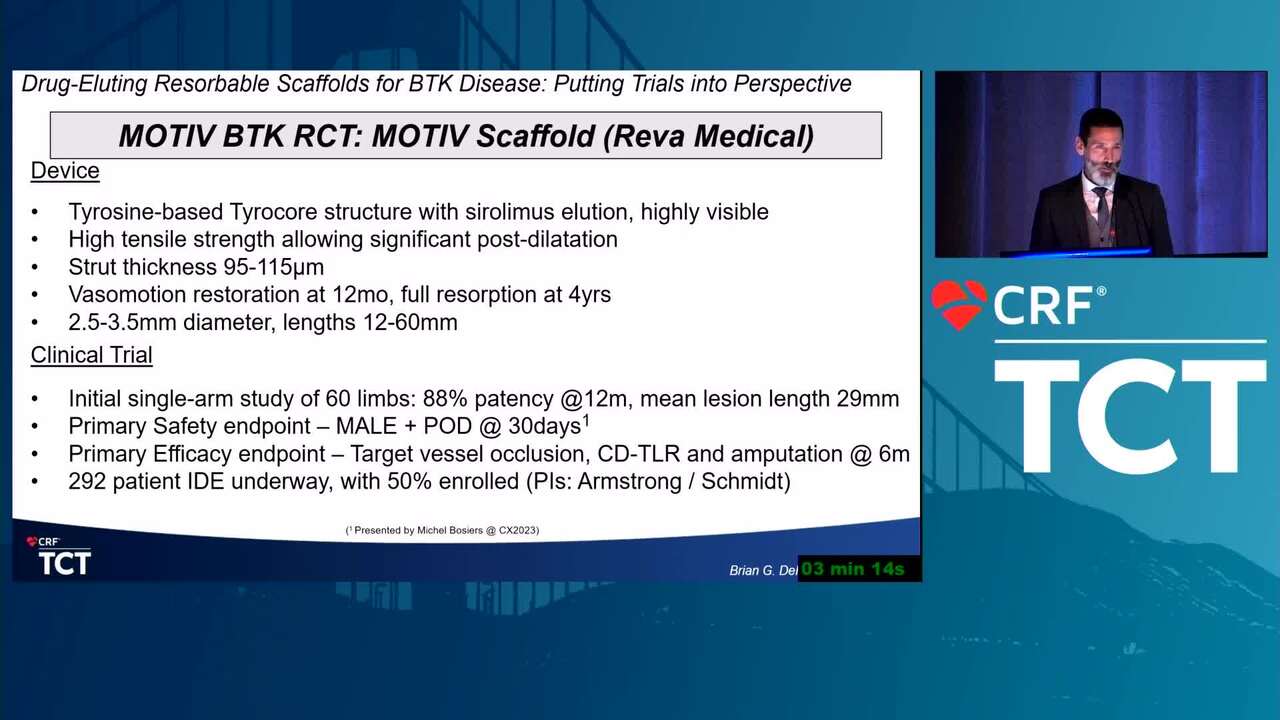
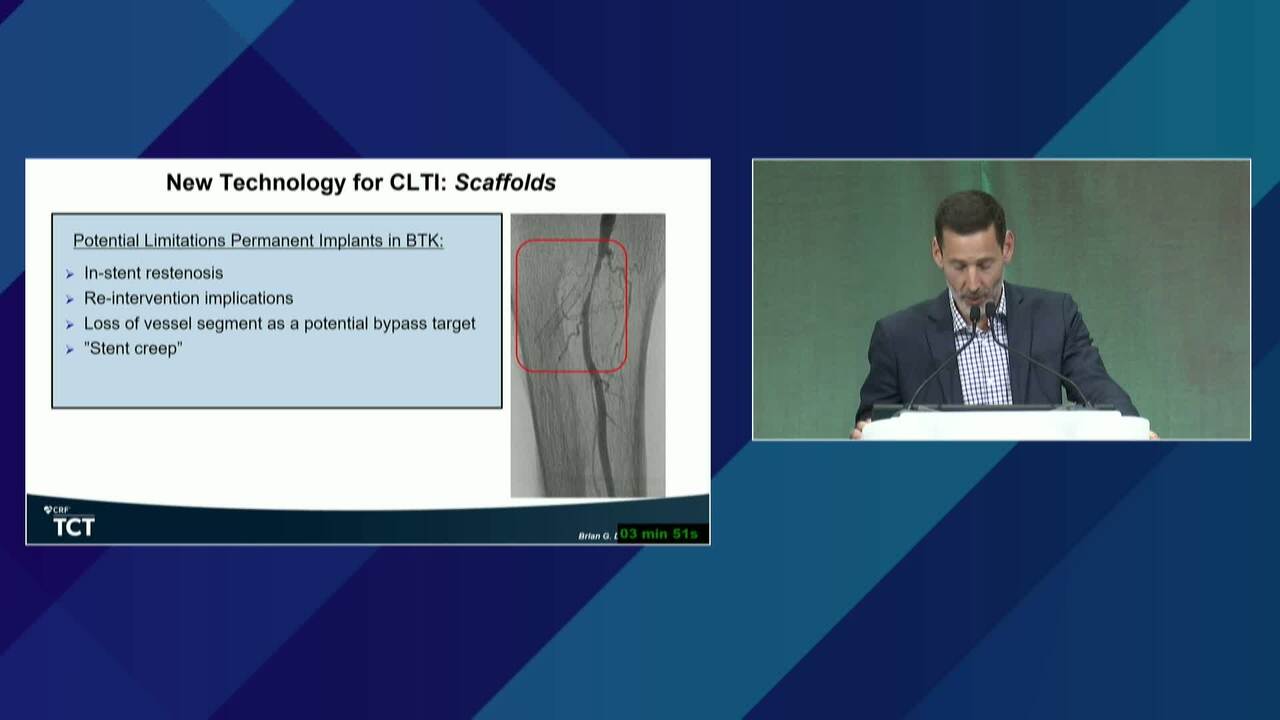
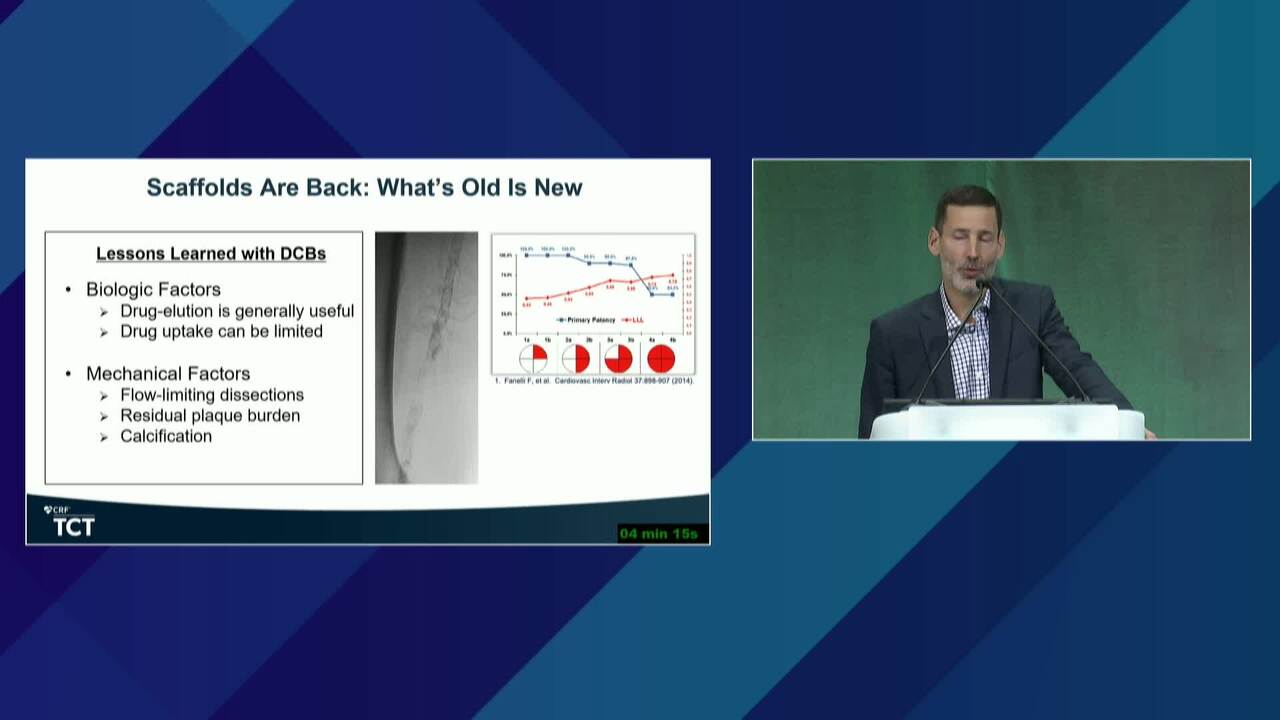
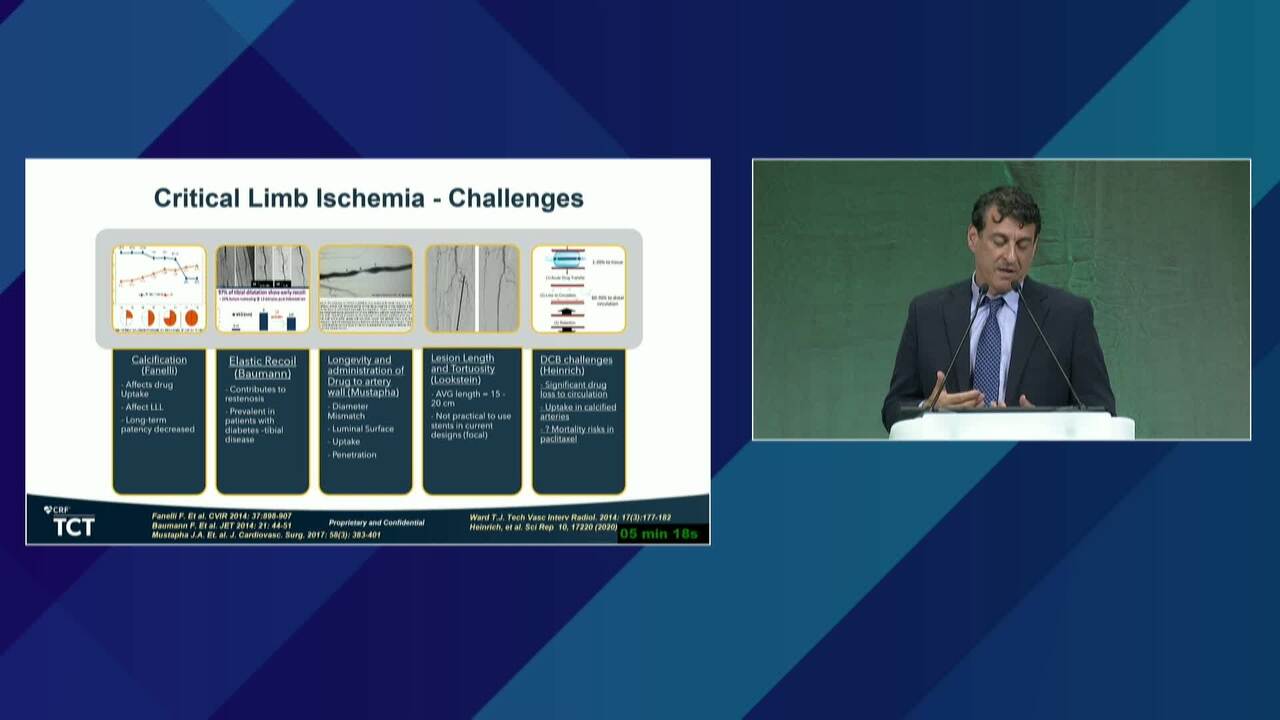
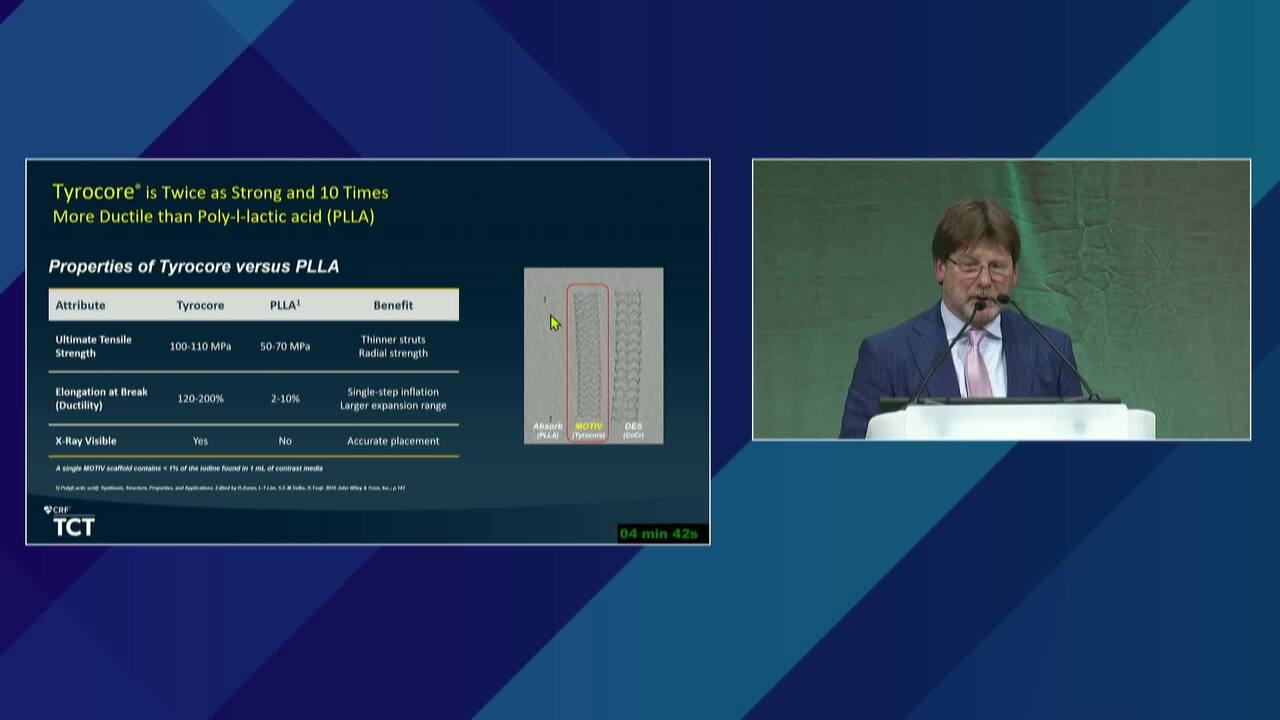
New BTK Bioresorbable Stent Technology: Life BTK, The MOTIV-BTK Trial and R3 Clinical Programs
10 min.
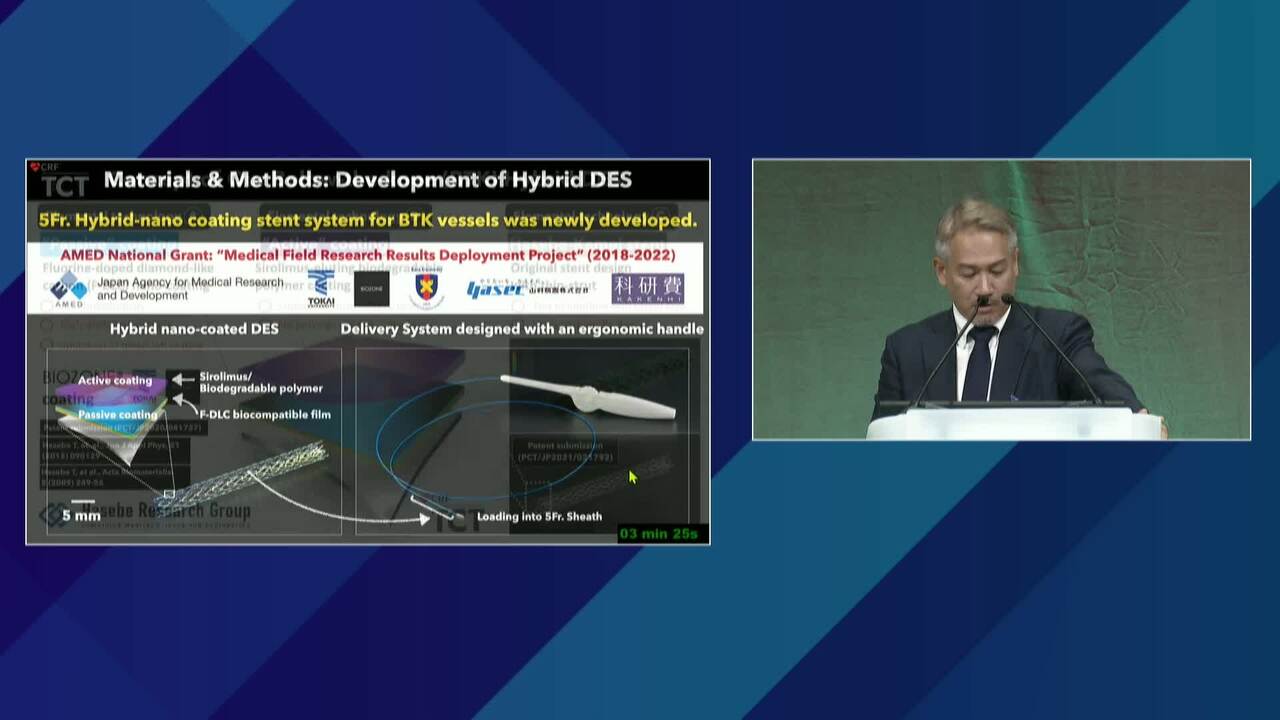
First Safety and Efficacy Study of the Misago Stent in Lower Extremity Endovascular Interventions via Radial Access Approach
8 min.
Sustained Limus Release vs Paclitaxel DCB Treatment in Symptomatic Peripheral Artery Disease: The Limus FLOW Investigator-Initiated, Randomized Controlled Trial
7 min.
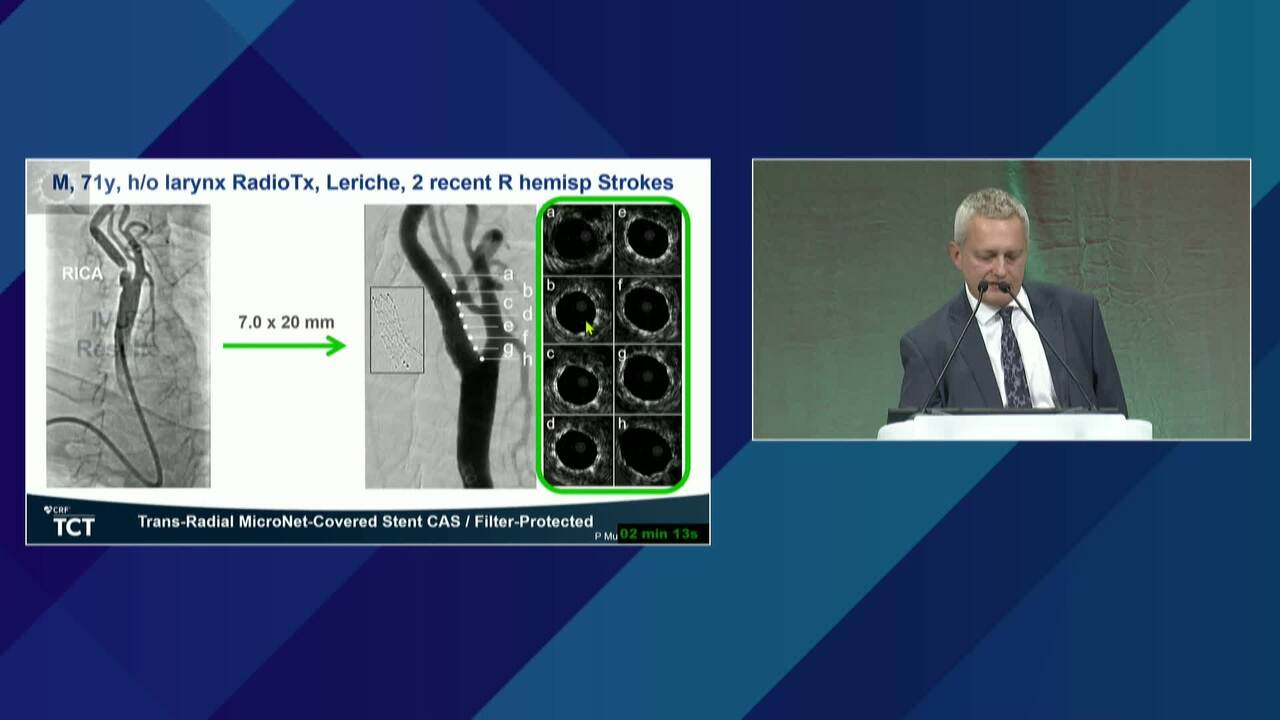
Endovascular Sequestration of High-Risk Carotid Lesions Using the MicroNET-Covered Embolic Prevention Stent in Consecutive Patients With Symptoms or Signs of Carotid Stenosis-Related Cerebral Injury: An Investigator-Initiated, Intravascular, Ultrasound-Controlled, Multinational, Multispecialty Trial (CGuard OPTIMA)
5 min.